Desperately seeking spin liquids, again
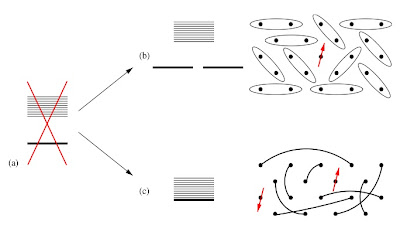
Previously I posted about recent numerical work which suggested that the Hubbard model on the honeycomb lattice and the Heisenberg model on the kagome lattice may have spin liquid ground states. At the Journal Club for Condensed Matter Physics , Steve Kivelson has a nice commentary on these two papers. One point important question he focuses on is whether or not these states have topological order , signified by a degenerate ground state, whose degeneracy only depends on the topology of the lattice [e.g. a torus].